Neurology & Rehabilitation Medicine
Services We Offer & Conditions We Treat:
- Comprehensive Stroke Center
- Epilepsy Center
- Headaches
- Lou Gehrig's Disease (ALS)
- Multiple Sclerosis
- Myasthenia Gravis Center
- Neuromuscular Disorders
- Peripheral neuropathy
- Myopathies
- Neurorehabilitation
- Spasticity Management
- Sports Medicine
- Traumatic Brain Injury
- Spinal Cord Injury
- Neck and Back Pain
- Parkinson's Disease (Movement Disorders Program)
- Sleep Disorders
- Transcranial magnetic stimulation (TMS) for Depression and Anxiety
The Department of Neurology & Rehabilitation Medicine at The George Washington University Medical Faculty Associates is a premier program that offers patients high quality, compassionate healthcare for conditions ranging from back pain and sleep disturbances to stroke and the most complex neuromuscular disorders.
Our medical team consists of board-certified neurologists, board-certified Physiatrists, nurses, EEG technicians, EMG technologists and clinical researchers. Our neurologists are on the forefront of advances in their fields with continuing education and by teaching at The George Washington University School of Medicine & Health Sciences. We offer fellowships in neurophysiology and sleep disorders.
To diagnose neuromuscular disorders we have invested in an advanced electrodiagnostic laboratory that has achieved the highest possible accreditation with exemplary status from the American Association of Neuromuscular & electrodiagnostic Medicine. Ours is the only lab in metropolitan Washington with this designation. It is proof of our commitment to clinical excellence and to providing the highest quality patient care.
We accept most insurance plans and our knowledgeable administrative staff is happy to assist with the approval process and pre-certifications.
Read the latest issue of Neurotransmitter: A magazine of the GW Institute for Neuroscience and the GW Hospital's Neurological Institute here.
Online Forms:
- Comprehensive Stroke Center
-
A stroke is a medical crisis that demands urgency, ideally in the form of ambulance transportation to the emergency room. When a blood vessel is blocked or ruptures, lack of oxygen causes brain cells to die. Immediate, skilled care is vital.
Our stroke center is certified and designated an Advanced Comprehensive Stroke Center by The Joint Commission. Certification as a Comprehensive Stroke Center means that the organization has met The Joint Commission’s standards for Disease-Specific Care Comprehensive Stroke Center Certification, making it part of an elite group of providers focused on complex stroke care. Complex Stroke Centers are recognized as industry leaders and are responsible for setting the national agenda in highly-specialized stroke care. These centers have advanced infrastructure, staff, and training to receive and treat patients with the most complex strokes.
In addition, the center has been honored by the American Heart Association and the American Stroke Association with several awards, including the Gold Quality Award and Get with the Guidelines. We are especially proud of our Target Stroke Award, which recognizes our ability to provide rapid, emergent treatment. Our stroke center is the only recipient of this award in metropolitan Washington.
The hallmark of our stroke care is consistency. The emergency room neurologist follows patients for the duration of their hospital stays and after discharge.

- Epilepsy Center
-
The specialists at The Epilepsy Center at The GW Medical Faculty Associates understand treatments should also be personalized. To accurately and effectively make a diagnosis, The Epilepsy Center provides inpatient and outpatient facilities equipped with imaging and electroencephalogram (EEG) technologies to map out the locations of abnormal brain activity. The Center also offers 24-hour video-EEG monitoring of seizures, so patients can quickly receive a diagnosis and begin treatment.
- Headache
-
Information coming soon.
- Lou Gehrig’s Disease (ALS)
-
Lou Gehrig’s disease involves the degeneration of nerve cells in the brain and spinal cord. It affects muscles involved in everything from limb movement to breathing.
To diagnose and treat affected patients, and to support their families and other caregivers, the John J. Kelly ALS Clinic at The GW Medical Faculty Associates deploys a collaborative, multidisciplinary team. It includes a board-certified neuromuscular neurologist, a board-certified pulmonologist, a neuromuscular nurse practitioner, and a clinical research coordinator as well as a speech-language pathologist, physical and occupational therapists, a dietitian, and a social worker who is an ALS specialist.
Members of the team are present at each visit; their different points of view are considered in each decision. Patients’ and caregivers’ opinions are respected as well, because our goals are the same: to help patients achieve the best possible quality of life.
Certified ALS Centers must meet strict criteria established by the National ALS Association and be involved in ALS research. Our researchers are involved in studies that offer promise in the fight against the disease.
- Multiple Sclerosis
-
Multiple sclerosis (MS) and other demyelinating disorders are major causes of neurological disability. Patients with MS suffer from a myriad of symptoms involving the motor, sensory, visual and cerebellar systems. Fatigue, behavioral symptoms and cognitive symptoms are recognized as serious components of the MS syndrome.
Our neurologists have extensive experience diagnosing and treating patients with multiple sclerosis by employing a carefully calibrated combination of medications, physical therapy, occupational therapy and, as appropriate, emerging experimental therapies.
As with any other chronic condition, our goal is to design a treatment plan that reduces symptoms and enhances quality of life. Recently developed pharmaceuticals significantly slow the progress of MS. The sooner these therapies are put in place, the better the outcome for both primary symptoms and potential complications.
- Myasthenia Gravis Center
-
Our mission at the Myasthenia Gravis Center (MGC) is to provide comprehensive state-of-the-art care to patients with MG through the collaboration of neurologists, surgeons, scientists, rehabilitation medicine therapists, and specialized consultative services.
- Neuromuscular Disorders
-
Our medical team provides comprehensive evaluation of patients with disorders of the peripheral nerves, muscles and the neuromuscular junction. We have neurologists that specialize in peripheral neuropathy, myopathies, Lou Gehrig’s disease (Amyotrophic Lateral Sclerosis) and myasthenia gravis. These specialists are internationally recognized for their expertise in diagnosis and management of these complex diseases of the muscles.
Advanced diagnostic strategies are critical in treatment planning for neuromuscular disorders. Laboratory tests include electromyography (EMG), Single Fiber-EMG, as well as routine and advanced nerve conduction studies. Muscle and skin biopsies are also performed to enhance the specificity of our diagnoses.
- Parkinson's Disease (Movement Disorders Program)
-
A committed, focused team of highly trained specialists in The Parkinson’s Disease and Movement Disorders Program cares for patients with Parkinson’s disease, tremors, dystonia, ataxia, chorea, restless leg syndrome, and tics, as well as functional and unusual movement disorders.
- Research
-
Our neurologists actively participate in clinical trials and have expertise in conducting research in a diverse array of neurological disorders including stroke, epilepsy, myasthenia gravis, peripheral neuropathy, amyotrophic lateral sclerosis, multiple sclerosis and movement disorders.
- Sleep
-
Tossing and turning night after night may not seem significant enough of a problem to see a neurologist, but inadequate sleep can lead to a host of serious medical problems, including high blood pressure and heart disease. If you regularly drive while drowsy, a car accident is not improbable. Fortunately, sleep problems have solutions.
The Center for Sleep Disorders at The GW Medical Faculty Associates incorporates modern technology with personal comfort in a secure, hotel-like setting. We pride ourselves on providing the finest sleep testing services available, seven nights a week. Patients are seen quickly and results are provided in a timely fashion.
Our center features an experienced, compassionate staff of board-certified physicians and polysomnographic technologists. In addition to the practice of sleep medicine, our team is also at the forefront of research, medical education, and the implementation of innovative treatment options.
- Transcranial Magnetic Stimulation (TMS)
-
Depression & Anxiety Treatment in Washington, DC
We are glad to offer transcranial magnetic stimulation (TMS) at The GW Medical Faculty Associates as a treatment of depression and anxiety.
Transcranial magnetic stimulation (TMS) is a noninvasive procedure that stimulates brain cells using magnetic fields to improve symptoms of depression and anxiety. TMS is usually used when other treatments for depression or anxiety such as medications and psychotherapy have failed.
During a TMS session, an electromagnetic coil is placed near your forehead on your scalp. In a painless way, the coil delivers pulses of magnetic fields stimulating brain cells involved in mood control, depression and anxiety.
Because the treatment for depression involves using repetitive pulses for each session, it is called repetitive TMS (rTMS).
What to Expect
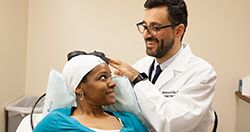
TMS sessions are carried out daily for 4-6 weeks. Each session will last for 20-40 minutes. The first session will typically last longer, around 60 minutes, as the physician has to determine the optimal site and the amount of magnetic energy needed for stimulation. This process is called mapping and is usually repeated every 2 weeks.
TMS is well tolerated and safe. However, some patients could experience some side effects.
Common side effects include: lightheadedness, headache, scalp discomfort, tingling or twitching of the facial muscles.
These side effects are usually mild and tend to improve with every subsequent session.
Serious side effects are very uncommon and include seizures, hearing loss (if there is inadequate ear protection during the sessions) and mania (in people with bipolar disorder).
You will need a referral from the physician treating your depression or anxiety and follow-up with the same physician to see if you are benefiting from the treatment.
What You Should Know
Symptom relief may take few weeks of treatment. Repetitive TMS (rTMS) is less likely to work if the mental illness includes psychotic symptoms, failure to respond to electroconvulsive therapy (ECT) and refractory chronic depression that has been lasting for many years.
Contraindications for TMS include the presence of any metallic or medical implanted device close enough to the electromagnetic coil such as aneurysm clips or coils and deep brain stimulators, pregnancy and a history of seizures.
If you suffer from depressive symptoms and are deemed a good candidate for rTMS by your referring physician, we look forward to seeing you in our TMS lab to help you.
Contact Us
Call (202) 677-6823 for more information.
How to Manage Back Pain During COVID-19
Dr. Akshay Garg and Dr. Geet Paul, Physical Medicine & Rehabilitation specialists discuss how to manage back pain during COVID-19. Please click on the 'play' video below.
Latest News
Mohamad Koubeissi, MD, MA, FAAN, FANA, FAES, professor of neurology, has been selected to serve as interim chair of the Department of Neurology and Rehabilitation Medicine. Koubeissi currently serves as the Director of the Epilepsy Center at the George Washington…
The George Washington University (GW) School of Medicine and Health Sciences (SMHS) recently announced a new name for its enterprise, the Department of Neurology & Rehabilitation Medicine.





