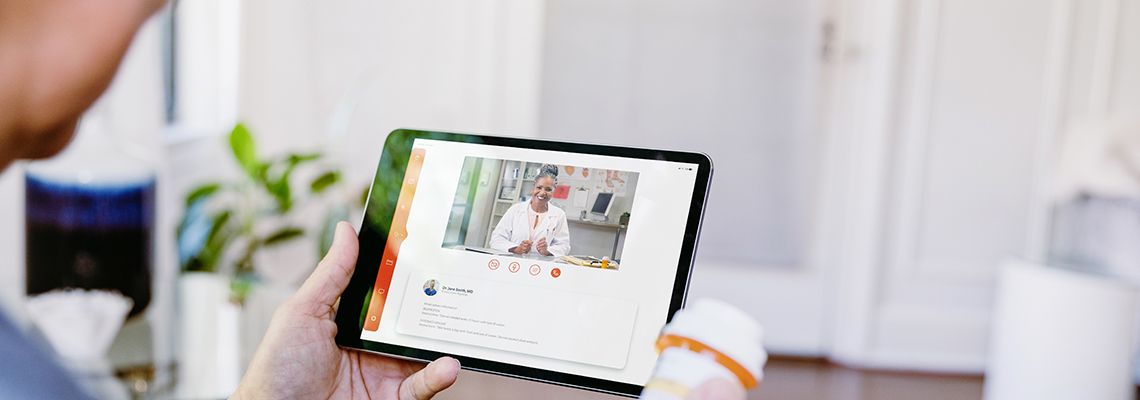
Schedule an Appointment With Your Urology Provider Virtually
The GW Medical Faculty Associates Urology Department is pleased to announce we are now offering Virtual Health Visits. Our virtual visits make access to our urology providers easier, faster and more convenient than ever for our patients. All you need is a computer, tablet or mobile phone with a webcam and access to the internet.
Virtual visits are possible for both NEW and EXISTING patients. Using telemedicine audiovisual communication, our GW Urology providers can assist you with the following:
- Assess your symptoms and order any diagnostic laboratory testing or imaging needed.
- Provide new patient visits, consults and second opinions
- Provide follow-up care with any treatments or surgeries
- Prescribe medications and medication refills
If it is determined that an in-person consultation or additional testing is required, then we will be happy to arrange that. Our scheduling staff will assist you in determining if you can be scheduled for a virtual appointment.
If you are interested in discussing the option to schedule a virtual visit and/or convert your existing upcoming appointment to a virtual visit, please contact our office at 202-741-3100 or please email: uroappointment@mfa.gwu.edu. Extended virtual health hours are coming soon.
Type of appointments that can be booked through virtual health care include conditions related to:
-
General Urology
-
Urologic cancers
-
Kidney stones
-
Reconstructive urology
-
Female urology/urogynecology
-
Male sexual health
For a full list of appointment types, please see the drop-down in the FAQs below.
Request a Virtual Health Visit
Please call (202) 741-3100 or email uroappointment@mfa.gwu.edu to schedule your virtual appointment.
Benefits of Virtual Health Appointments
- No parking or transportation hassles.
- No wait times.
- More time spent face-to-face with the doctor.
- Easy to do – once scheduled you will receive a Zoom link to your appointment. Zoom is easily downloaded from the Apple App store or Google Play store and is HIPAA compliant.
- Most insurances accepted. (Please check your virtual/telehealth benefits with your insurance plan)
- All new evaluations triaged to most qualified specialist to address the problem
Frequently Asked Questions
- How do virtual health visits work?
-
We use the secure video platform, Zoom, a health information (HIPAA) compliant video conferencing system that allows you to have a video conference-based visit with your GW Urology physician or provider. You will need to have a computer, tablet or smart phone with a front facing camera to participate in the virtual health visit.
The Zoom application is available for download to use on smart phones and tablets. The apps are available in the Apple App Store and on Google Play. See instructions below for steps to set up your computer, tablet or phone for Zoom. Zoom is a free video communications software and there is no charge for using Zoom.
- What are the next steps after downloading the software?
-
- Once you have downloaded the software, a box will prompt you to enter your name. Please enter your first and last name.
- The next prompt will ask you how you’d like the audio (sound source) for the meeting to be connected. It is best to connect by computer or the audio on your tablet or smart phone.
- Be sure that your microphone is not muted and that your speakers are not muted. This way you and your provider can speak to each other. You will also need to make sure that your video stream is “started” so that you can see each other. You may have to hover your mouse over the screen on your computer or tap the screen on your phone to get this toolbar to appear.
- You should be able to see and talk to your provider. At the beginning of your appointment, your provider will also ask you for an alternate contact number at which to reach you, just in case you have trouble getting started or if you get disconnected during the appointment.
This set up does not have to be done again for subsequent visits.
- Is this service covered by insurance?
-
Online care is covered by Medicare and most insurance plans.
- Is my personal health information kept confidential?
-
Yes! Our online urology care platform is secure and 100% HIPAA and HITECH compliant.
- What urologic conditions can be treated with a virtual appointment?
-
Type of appointments that can be booked through virtual health care include:
General Urology
- Benign Prostatic Enlargement (BPH)
- Bothersome urinary symptoms
- Diagnostic and Laboratory Results Follow-up
- Urinary Tract Infections
- Blood in the Urine (Hematuria)
- Second Opinions & Consults
Urologic Cancers
- Prostate Cancer Treatment
- Prostate Cancer PSA Screening
- Kidney Mass & Kidney Cancer
- Bladder Mass & Bladder Cancer
- Testis Cancer
Kidney Stones
- Metabolic management
- Surgical treatments
Reconstructive Urology
- Urethral stricture
- Repair of traumatic injury
- Transition from Pediatric to Adult Urology
- Female and Male Incontinence
- LGBTQ Specialized Services: Compassionate Care, Gender Affirmation Surgery
Female Urology/ Urogynecology
- Pelvic Organ Prolapse (Bladder/Uterus)
- Women’s Sexual Health
- Pelvic Floor Disorders
- Urinary incontinence
- Postpartum Pelvic Floor Evaluation
Male Sexual Health
- Male Fertility Evaluations and Care
- Vasectomy Reversal
- Low Testosterone
- Male Pelvic Pain
- Erectile Dysfunction
